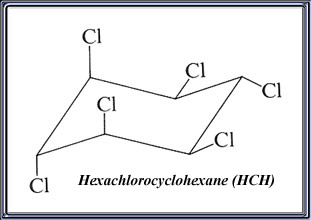
Lindane Lindane is an organochlorine insecticide which has been used on a wide range of soil-dwelling and plant-eating (phytophagous) insects. It is commonly used on a wide variety of crops, in warehouses, in public health to control insect-borne diseases, and (with fungicides) as a seed treatment. Lindane is also presently used in lotions, creams, and shampoos for the control of lice and mites (scabies) in humans.Technical lindane is comprised of the gamma-isomer of hexachlorocyclohexane, HCH. Five other isomers (molecules with a unique structural arrangement, but identical chemical formulas) of HCH are commonly found in technical lindane, but the gamma-isomer is the predominant one, comprising at least 99% of the mixture of isomers. Data presented in this profile are for the technical product unless otherwise stated; lindane, HCH, or BHC refer to technical lindane, i.e., Gamma-hexachlorocyclohexane. Gamma-HCH has been shown to be the insecticidally effective isomer.
Lindane may also be found in formulations with a host of fungicides and insecticides. It is available as a suspension, emulsifiable concentrate, fumigant, seed treatment, wettable and dustable powder, and ultra low volume (ULV) liquid.
IUPAC Name: gamma 1α,2α,3β,4α,5α,6β-hexachlorocyclohexane
Chemical Formula: C6H6Cl6
NOTE: Lindane (or hexachlorocyclohexane, HCH) has historically and widely been inappropriately referred to as "benzene hexachloride" or "BHC". This compound should not be confused with hexachlorobenzene, or HCB.
LD50/LC50: Lindane is a moderately toxic compound via oral exposure, with a reported oral LD50 of 88 to 190 mg/kg in rats. Other reported oral LD50 values are 59 to 562 mg/kg in mice, 100 to 127 mg/kg in guinea pigs, and 200 mg/kg in rabbits. Gamma-HCH is generally considered to be the most acutely toxic of the isomers following single administration. It is moderately toxic via the dermal route as well, with reported dermal LD50 values of 500 to 1000 mg/kg in rats, 300 mg/kg in mice, 400 mg/kg in guinea pigs, and 300 mg/kg in rabbits. Effects of high acute exposure to lindane may include central nervous system stimulation (usually developing within 1 hour), mental/motor impairment, excitation, clonic (intermittent) and tonic (continuous) convulsions, increased respiratory rate and/or failure, pulmonary edema, and dermatitis. Other symptoms in humans are more behavioral in nature such as loss of balance, grinding of the teeth, and hyper-irritability. Most acute effects in humans have been due to accidental or intentional ingestion, although inhalation toxicity occurred (especially among children) when it was used in vaporizers. Workers may be exposed to the product through skin absorption and through inhalation if handled incorrectly. Lotions (10%) applied for scabies have resulted in severe intoxication in some children and infants. It is reported that single administrations of 120 mg/kg inhibited the ability of white blood cells to attack and kill foreign bacteria in the blood of rats, and 60 mg/kg inhibited antibody formation to human serum albumin. It is not clear whether these effects were temporary, or for how long they may have lasted.
Effects on birds: Lindane is moderately to practically nontoxic to bird species, with a reported LD50 of more than 2000 mg/kg in the mallard duck. The LC50 values of lindane in pheasant and bobwhite quail are 561 ppm and 882 ppm, respectively. Egg-shell thinning and reduced egg production has occurred in birds exposed to lindane. Lindane can be stored in the fat of birds; birds of prey in the Netherlands contained up to 89 ppm in their fat. Residues can also find their way into egg yolks at measurable concentrations for 32 days after dosing.
Effects on aquatic organisms: Lindane is highly to very highly toxic to fish and aquatic invertebrate species. Reported 96-hour LC50 values range from 1.7 to 90 ug/L in trout (rainbow, brown, and lake), salmon, carp, largemouth bass. Water hardness did not seem to alter the toxicity to fish, but increased temperature caused increased toxicity for some species and decreased toxicity for others. Reported 96-hour LC50 values in aquatic invertebrates were: in Daphnia, 460 ug/L; in scuds, 10-88 ug/L; and in Pteronarcys (stone flies), 4.5 ug/L. The bioconcentration factor for the compound is 1400 times ambient water concentrations, indicating significant bioaccumulation.
Effects on other organisms: ***Lindane is highly toxic to bees***.
Breakdown in soil and groundwater: Lindane is highly persistent in most soils, with a field half-life of approximately 15 months. When sprayed on the surface, the half-life was typically much shorter than when incorporated into the soil. It shows a low affinity for soil binding, and may be mobile in soils with especially low organic matter content or subject to high rainfall. It may pose a risk of groundwater contamination. The pesticide has been found in a significant number of groundwater samples in the USA and Italy.
Breakdown in water: Lindane is very stable in both fresh and salt water environments, and is resistant to photodegradation. It will disappear from the water by secondary mechanisms such as adsorption on sediment, biological breakdown by microflora and fauna, and adsorption by fish through gills, skin, and food.
Breakdown in vegetation: Plants may pick up residues from not only direct application, but through water and vapor phases. Persistence is seen when plants are rich in lipid content, and crops like cauliflower and spinach will build up less residue than crops like carrots. The metabolism in plants is not well understood, but carrots are estimated to metabolize lindane with a half-life of just over 10 weeks (based on plant uptake) whereas it may have a half-life in lettuce of only 3 to 4 days.
Physical Properties:
- Appearance: Lindane is a colorless crystal compound.
- Chemical Name: gamma-1,2,3,4,5,6-hexachlorocyclohexane.
- CAS Number: 58-89-9.
- Molecular Weight: 290.85.
- Water Solubility: 7.3 mg/L @ 25°C.
- Solubility in Other Solvents: v.s. in acetone, benzene, and ethanol.
- Melting Point: Approximately 113°C.
- Vapor Pressure: 5.6 mPa @ 20°C.
- Partition Coefficient: Not Available.
- Adsorption Coefficient: 1100.
Sources: Oregon University and The World Health Organisation