Chlordane Chlordane is a persistent organochlorine insecticide. It kills insects when ingested and on contact. Formulations include dusts, emulsifiable concentrates, granules, oil solutions, and wettable powders.IUPAC Name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methanoindene
Chemical Formula: C10H6Cl8
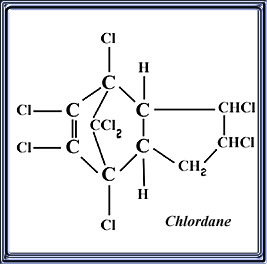
And this is what it looks like
LD50/LC50: The oral LD50 for chlordane in rats is 200 to 700 mg/kg, in mice is 145 to 430 mg/kg, in rabbits is 20 to 300 mg/kg, and in hamsters is 1720 mg/kg. The dermal LD50 in rabbits is 780 mg/kg, and in rats is 530 to 690 mg/kg. The 4-hour inhalation LD50 in cats is 100 mg/L.
Chlordane is moderately to highly toxic through all routes of exposure. Symptoms usually start within 45 minutes to several hours after exposure to a toxic dose. Convulsions may be the first sign of poisoning, or they may be preceded by nausea, vomiting, and gut pain. Initially, poisoning victims may appear agitated or excited, but later they may become depressed, uncoordinated, tired, or confused. Other symptoms reported in cases of chlordane poisoning include headaches, dizziness, vision problems, irritability, and weakness or muscle twitching. In severe cases, respiratory failure and death may occur. Complete recovery from a toxic exposure to chlordane is possible if proper medical treatment is administered. Chlordane is very irritating to the skin and eyes. Chlordane affects liver function, so many interactions between medicines and this pesticide may occur. Among these are decreased effectiveness of anticoagulants, phenylbutazone, chlorpromazine, steroids, birth control pills, and diphenhydramine. Increased activity of thyroid hormone may also occur.
In human beings chlordane is absorbed into the body through the lungs, stomach, and skin. It is stored in fatty tissues as well as in the kidneys, muscles, liver, and brain. Chlordane has been found in human fat samples at concentrations of 0.03 to 0.4 mg/kg in U.S. residents. Chlorinated hydrocarbons stored in fatty tissues can become released into circulation if these fatty tissues are metabolized, as in starvation or intense activity. Chlordane that is not stored in the body is excreted through the urine and feces. Chlordane has been found in human breast milk. Rats that breathed chlordane vapor for 30 minutes retained 77% of the total amount inhaled. Rabbits that received 4 doses of chlordane stored it in fatty tissues, the brain, kidneys, liver, and muscles. Excretion of orally administered chlordane is slow and can take days to weeks. The biological half-life of chlordane in the blood serum of a 4-year-old child who drank an emulsifiable concentrate of chlordane was 88 days. In another accidental poisoning of a 20-month-old child, the half-life was 21 days.
Effects on birds: Chlordane is moderately to slightly toxic to birds. The 8-day dietary LC50 for chlordane is 858 ppm in mallard ducks and 430 ppm in pheasant.
Effects on aquatic organisms: Chlordane is very highly toxic to fresh water invertebrates and fish. The LC50 (96-hour) for chlordane in bluegill is 0.057 to 0.075 mg/L and 0.042 to 0.090 mg/L in rainbow trout. Chlordane bioaccumulates in bacteria and in marine and freshwater fish species. Expected bioaccumulation factors for chlordane are in excess of 3000 times background water concentrations indicating that bioconcentration is significant for this compound.
Effects on other organisms: Chlordane is highly toxic to bees and earthworms. Studies done in the late 1970s showed that the fatty tissues of land and water wildlife contained large amounts of cyclodiene insecticides, including chlordane.
Breakdown in soil and groundwater: Chlordane is highly persistent in soils with a half-life of about 4 years. Several studies have found chlordane residues in excess of 10% of the initially applied amount 10 years or more after application. Sunlight may break down a small amount of the chlordane exposed to light. Evaporation is the major route of removal from soils. Chlordane does not chemically degrade and is not subject to biodegradation in soils. Despite its persistence, chlordane has a low potential for groundwater contamination because it is both insoluble in water and rapidly binds to soil particles making it highly immobile within the soil. Chlordane molecules usually remain adsorbed to clay particles or to soil organic matter in the top soil layers and slowly volatilize into the atmosphere. However, very low levels of chlordane (0.01 to 0.001 ug/L) have been detected in both ground and surface waters in areas where chlordane was heavily used. Sandy soils allow the passage of chlordane to groundwater.
Breakdown in water: Chlordane does not degrade rapidly in water. It can exit aquatic systems by adsorbing to sediments or by volatilization. The volatilization half-life for chlordane in lakes and ponds is estimated to be less than 10 days. Chlordane has been detected in surface water, groundwater, suspended solids, sediments, bottom detritus, drinking water, sewage sludge, and urban run-off, but not in rain water. Concentrations detected in surface water have been very low, while those found in suspended solids and sediments are always higher (<0.03 to 580 ug/L). The presence of chlordane in drinking water has almost always been associated with an accident rather than with normal use.
Breakdown in vegetation: No data are currently available.
Physical Properties:
- Appearance: Technical chlordane is actually a mixture of at least 23 different components, including chlordane isomers, other chlorinated hydrocarbons, and by-products. It is a viscous, colorless or amber-colored liquid with a chlorine-like odor.
- Chemical Name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methanoindene.
- CAS Number: 57-74-9.
- Molecular Weight: 409.83.
- Water Solubility: 0.1 mg/L @ 25°C.
- Solubility in Other Solvents: s. in most organic solvents, including petroleum oils.
- Melting Point: 104-107°C.
- Vapor Pressure: 1.3 mPa @ 25°C.
- Partition Coefficient: 2.78.
- Adsorption Coefficient: 20,000.
Sources: Oregon University and The World Health Organisation